22.10.2024
Letter of Announcement: EU Hub Release 1.16: Reminder of deployment dates & Upcoming changes workshops
01.10.2024
EMVO LoA: EU Hub Release 1.16: Reminder of deployment dates & Upcoming changes workshops
12.09.2024
Letter of Announcement: EU Hub Release 1.16: Planned deployment dates for ITE, IQE & PRD
01.08.2024
01/08/2024 Letter of Announcement: Availability of EMVO Support during the August bank holiday
30.07.2024
30/07/2024 Letter of Announcement: Microsoft network issues
19.07.2024
19/07/2024 Letter of Announcement: Global IT outage affecting Microsoft
02.07.2024
02/07/2024 Letter of Announcement: EU Hub Release 1.15: Confirmation of deployment to PRD
27.06.2024
27/06/2024 Letter of Announcement: OBP Portal 2024: Benefits & training sessions
26.06.2024
26/06/2024 Letter of Announcement: EU Hub Release 1.15: Reminder of deployment date to PRD
17.06.2024
17/06/2024 Letter of Announcement: EU Hub Release 1.15: Change of deployment date to PRD
11.06.2024
10/06/2024 Letter of Announcement: EU Hub Release 1.15: Confirmation of deployment date to PRD
15.05.2024
13/05/2024 Letter of Announcement: EU Hub Release 1.15: Confirmation of deployment to ITE & IQE
14.05.2024
14/05/2024 Letter of Announcement: EMVO LoA: OBP Portal 2024: Q&A session June
07.05.2024
07/05/2024 Letter of Announcement: Reminder: Consequences of Product Withdrawal & Batch recall
02.05.2024
02/05/2024 Letter of Announcement: EU Hub Release 1.15: New change of deployment dates for ITE & IQE
25.04.2024
25/04/2024 Letter of Announcement: Availability of EMVO Support during the upcoming bank holidays
23.04.2024
23/04/2024 Letter of Announcement: OBP Portal 2024 Update and Migration
22.04.2024
22/04/2024 Letter of Announcement: EU Hub Release 1.15: Change of deployment dates for ITE & IQE
22.04.2024
25/03/2024 Letter of Announcement: EU Hub Release 1.15: Planned deployment dates for ITE, IQE & PRD
22.04.2024
03/04/2024 Letter of Announcement: Maintenance of OBP Contact Details
25.03.2024
25/03/2024 Letter of Announcement: EU Hub Release 1.15: Planned deployment dates for ITE, IQE & PRD
18.03.2024
18/03/2024 Letter of Announcement: Availability of EMVO Support during the upcoming Easter holiday
25.01.2024
25/01/2024 Letter of Announcement: AMS Hub and AMS Portal downtime
24.01.2024
22/01/2024 Letter of Announcement: EU Hub Release 1.14 Confirmation of deployment to PRD
09.01.2024
08/01/2024 Letter of Announcement: EU Hub Release 1.14: Confirmation of deployment date for PRD
03.01.2024
11/12/2023 Letter of Announcement: EMVO Support & Helpdesk – 2023 Holiday season availability
03.01.2024
27/11/2023 Letter of Announcement: AMS Clementine – Confirmation of deployment
20.11.2023
20/11/2013 Letter of Announcement: System Supplier Change in Finland – Confirmation
10.11.2023
10/11/2023 Letter of Announcement: AMS Clementine Release – Postponement of deployment to PRD
07.11.2023
07/11/2023 Letter of Announcement: System Supplier Change in Finland – Reminder
03.11.2023
03/11/2023 Letter of Announcement: NMVO Fee Models Overview for 2024
30.10.2023
30/10/2023 Letter of Announcement: EU Hub Release 1.14: Confirmation of deployment to IQE
26.10.2023
26/10/223 Letter of Announcement: EU Hub Release 1.14: Confirmation of deployment to ITE
25.10.2023
25/10/2023 Letter of Announcement: Availability of EMVO Support during upcoming bank holiday
24.10.2023
24/10/2023 Letter of Announcement: #Alerts filtering
23.10.2023
23/10/2023 Letter of Announcement: EU Hub Release 1.14: Postponement of deployment to ITE
20.10.2023
20/10/2023 Letter of Announcement: Resolved – Microsoft performance issues
20.10.2023
20/10/2023 Letter of Announcement: Microsoft performance issues
18.10.2023
18/10/2023 Letter of Announcement: System Supplier Change in Finland
11.10.2023
11/10/2023 Letter of Announcement: EU Hub Release 1.14: Planned deployment dates for ITE, IQE & PRD
11.09.2023
11/09/2023 Letter of Announcement: Resolved – Missing “distributed” callbacks when uploading PPD
17.07.2023
17/07/2023 Letter of Announcement: Availability of EMVO Support during the upcoming summer holiday
14.07.2023
14/07/2023 Letter of Announcement: Missing “distributed” callbacks when uploading PPD
10.07.2023
10/07/2023 Letter of Announcement: New EMVO Webcast Series Episodes
26.06.2023
26/06/2023: Letter of Announcement: EU Hub Release 1.13: Confirmation of deployment to PRD
21.06.2023
21/06/2023: Letter of Announcement: EU Hub Release 1.13: Reminder for deployment to PRD
16.06.2023
16/06/2023: Letter of Announcement: EAMS Upcoming Releases in 2023 – AMS Cybersecurity 1
14.06.2023
14/06/2023: Letter of Announcement: EAMS Upcoming Releases in 2023 – AMS Clementine
07.06.2023
07/06/2023: Letter of Announcement: EU Hub Release 1.13: Confirmation of deployment date for PRD
31.05.2023
31/05/2023: Letter of Announcement: Reminder: Consequences of product withdrawal & batch recall
12.05.2023
12/05/2023: Letter of Announcement: Changes to the NMVO Observers
12.05.2023
05/05/2023: Letter of Announcement: EU Hub Release 1.13: Confirmation of deployment to IQE
02.05.2023
02/05/2023: Letter of Announcement: EAMS Upcoming Releases in 2023
25.04.2023
25/04/2023: Letter of Announcement: EU Hub Release 1.13: Postponement of deployment to IQE
25.04.2023
25/04/2023: Letter of Announcement: EU Hub Release 1.13: Confirmation of deployment to ITE
24.04.2023
24/04/2023: Letter of Announcement: Availability of EMVO Support during the upcoming bank holidays
03.04.2023
03/04/2023: Letter of Announcement: Availability of EMVO Support during the upcoming spring holidays
30.03.2023
30/03/2023: Letter of Announcement: EU Hub Release 1.13 Planned deployment dates for ITE IQE PRD
28.03.2023
28/03/2023: Letter of Announcement: Static IP addresses EU Hub
24.03.2023
24/03/2023: Letter of Announcement: Removal of old ciphers with EU Hub Release 1.13
16.03.2023
16/03/2023: Letter of Announcement: Validity of Private Certificates
01.03.2023
01/03/2023: Letter of Announcement: EAMS officially live & upcoming releases
01.03.2023
01/03/2023: Letter of Announcement: Changes to EMVO Board of Directors
09.02.2023
09/02/2023: Letter of Announcement: EAMS officially live & next steps
09.02.2023
09/02/2023: Letter of Announcement: The European Medicines Verification System’s 4th year anniversary
06.02.2023
06/02/2023: Letter of Announcement: EAMS “Go-live” date & next steps
25.01.2023
25/01/2023: Letter of Announcement: Microsoft performance issue RESOLVED
25.01.2023
25/01/2023: Letter of Announcement: Microsoft performance issue detected
23.01.2023
23/01/2023: Letter of Announcement: EU Hub Release 1.12 Confirmation of deployment to PRD
15.12.2022
15/12/2022: Letter of Announcement: EMVO Support & Helpdesk: Holiday season availability
15.12.2022
14/12/2022: Letter of Announcement: Update on OBP Portal log-in
12.12.2022
12/12/2022: Letter of Announcement: Webcast Series – EMVO Gateway training
06.12.2022
06/12/2022: Letter of Announcement – OBP D&A Guide Update
28.11.2022
28/11/2022 Letter of Announcement: EU Hub Release 1.12: Confirmation of deployment date for PRD
24.11.2022
24/11/2022 Letter of Announcement: EMVS Master Data Guide Update
23.11.2022
22/11/2022 Letter of Announcement: OBP Portal log-in
18.11.2022
18/11/2022 Letter of Announcement: Performance issue impacting the EU Hub: RESOLVED
10.11.2022
10/11/2022 Letter of Announcement: Helpdesk phone line restored
09.11.2022
09/11/2022 Letter of Announcement: Temporary limited availability reaching the helpdesk by phone
08.11.2022
08/11/2022 Letter of Announcement: Stabilisation Period Overview Update
07.11.2022
07/11/2022 Letter of Announcement: EU Hub Release 1.12: Confirmation of deployment to IQE
28.10.2022
28/10/2022 Letter of Announcement: Availability of EMVO Support during the upcoming bank holidays
27.10.2022
27/10/2022 Letter of Announcement: EU Hub Release 1.12: Confirmation of deployment to ITE
25.10.2022
25/10/2022 Letter of Announcement: EU Hub Release 1.12: New deployment date for ITE
21.10.2022
21/10/2022 Letter of Announcement: EU Hub Release 1.12: Delay of deployment to ITE
20.09.2022
20/09/2022 Letter of Announcement: Temporary limited capacity of EMVO’s helpdesk
19.09.2022
19/09/2022 Letter of Announcement: NMVO Fee Models Overview for 2023
13.09.2022
13/09/2022 Letter of Announcement: EMVO launches the Self-Service Portal
08.09.2022
08/09/2022 Letter of Announcement: EMVO EU Hub performance issues RESOLVED
07.09.2022
07/09/2022 Letter of Announcement: EMVO EU Hub performance issues
02.09.2022
02/09/2022 Letter of Announcement: EMVO OBP Portal issue update
02.09.2022
02/09/2022 Letter of Announcement: EMVO OBP Portal temporarily unavailable
02.09.2022
02/09/2022 Letter of Announcement: EU Hub Release 1.12 Planned Deployment Dates for ITE IQE PRD
17.08.2022
17/08/2022 Letter of Announcement: Small Markets (Brexit) FAQ
09.08.2022
09/08/2022 Letter of Announcement: AMS Lilith Demo
29.07.2022
29/07/2022 Letter of Announcement: Status of packs intended for the Greek & Italian markets
18.07.2022
18/07/2022 Letter of Announcement: Availability of EMVO support during the upcoming bank holidays
07.07.2022
07/07/2022 Letter of Announcement: European Commission Q&A Version 20
28.06.2022
28/06/2022 Letter of Announcement: Renewal EU Hub Certificates confirmation deployment
23.06.2022
23/06/2022 Letter of Announcement: Reminder: Renewal of EU Hub Public Certificates & alert endpoints
20.06.2022
20/06/2022 Letter of Announcement: EMVO Chief Operating Officer
15.06.2022
15/06/2022 Letter of Announcement: RESOLVED: Arvato National Systems Temporary Unavailability
15.06.2022
15/06/2022 Letter of Announcement: Update: Renewal of EU Hub Public Certificates & alert endpoints
13.06.2022
11/06/2022 Letter of Announcement: EU Hub Release 1.11: Confirmation of deployment to PRD
10.06.2022
10/06/2022 Letter of Announcement: Alert Management System – End-to-End demonstration
07.06.2022
07/06/2022 Letter of Announcement: EU Hub Release 1.11 Confirmation of deployment date to PRD
31.05.2022
31/05/2022 Letter of Announcement: Update – Renewal of EU Hub Public Certificates & alert endpoints
20.05.2022
20/05/2022 Letter of Announcement: Availability of EMVO Support during the upcoming bank holidays
19.05.2022
19/05/2022 Letter of Announcement: Update – Renewal of EU Hub Public Certificates & alert endpoints
16.05.2022
11/05/2022 Letter of Announcement: Update – Renewal of EU Hub Public Certificates & alert endpoints
10.05.2022
09/05/2022 Letter of Announcement: Webcast Series – OBP Data Upload Quality
10.05.2022
28/04/2022 Letter of Announcement: EU Hub Release 1.11: Confirmation of deployment to IQE
26.04.2022
26/04/22 Letter of Announcement: EU Hub Release 1.11 – Confirmation of deployment to ITE
26.04.2022
26/04/2022 Letter of Announcement: Renewal of EU Hub Public Certificates & alert endpoints
22.04.2022
22/04/2022 Letter of Announcement: EU Hub Release 1.11 Planned deployment dates for ITE, IQE & PRD
13.04.2022
13/04/2022 Letter of Announcement: Support and Helpdesk Holiday Availability
05.04.2022
2022/04/05 Letter of Announcement: Renewal EU Hub Certificates & Alert endpoints
21.03.2022
2022/03/21 Letter of Announcement: Maintenance OBP of Contact Details
09.03.2022
2022/03/09 Letter of Announcement: Whitelist additional EU Hub IP addresses in your firewall
07.03.2022
2022/02/28 Letter of Announcement: Upload to UKNI of serialised packs
01.02.2022
2022/02/01 Letter of Announcement – New AMS Info Sessions and AMS Pilot Rollout for OBPs
18.01.2022
2022/01/17 Letter of Announcement: European Commission Q&A Version 19
15.12.2021
2021/12/15 Letter of Announcement: EMVO Support & Helpdesk: Holiday season availability
14.12.2021
2021/12/14 Letter of Announcement: Incident on Alert Messages for OBPs – Resolved
13.12.2021
2021/12/13 Letter of Announcement: Reminder on mandatory conformity to Transport Layer Security (TLS) 1.2 or higher
10.12.2021
2021/12/10 Letter of Announcement: Incident on Alert Messages for OBPs
07.12.2021
2021/12/07 Letter of Announcement: EMVO Participation Agreement: Notice Contact Update
06.12.2021
2021/12/06 Letter of Announcement: EU Hub Release 1.10: Confirmation of deployment to PRD
25.11.2021
2021/11/25 Letter of Announcement: EU Hub Release 1.10: Deployment date for PRD
22.11.2021
2021/11/22 Letter of Announcement: COO-Tobias Beer
03.11.2021
2021/11/03 Letter of Announcement: EU Hub Release 1.10: Confirmation of deployment to IQE
28.10.2021
2021/10/28 Letter of Announcement: EU Hub Release 1.10: Confirmation of deployment to ITE
21.10.2021
2021/10/21 Letter of Announcement: EU Hub Release 1.10: Planned deployment dates for ITE, IQE & PRD
13.10.2021
2021/10/13 Letter of Announcement: AMS Pilot and Info Sessions
29.09.2021
2021/09/29 Letter of Announcement: Verifications of packs under physical possession of an OBP
21.09.2021
2021/09/21 Letter of Announcement: Irreversible transactions
20.09.2021
2021/09/20 Letter of Announcement: Alert Management System Updates
03.09.2021
2021/09/03 Letter of Announcement: NMVO Fee Models Overview 2022
02.09.2021
2021/09/02 Letter of Announcement: Alert Management System updates
06.08.2021
2021/08/06 Letter of Announcement: Webcast Series Launch – EU FMD Designated Wholesalers
02.08.2021
2021/08/02 Letter of Announcement: Alert processing management: A2 & A52 alerts triggered by the EU Hub with EU Hub Release 1.10
27.07.2021
2021/07/27 Letter of Announcement: Mandatory conformity to Transport Layer Security (TLS) 1.2 or higher
12.07.2021
2021/07/12 Letter of Announcement: Important reminder about Designated Wholesalers
23.06.2021
2021/06/23 Letter of Announcement: “Best Practice on Alert Handling” Guideline
22.06.2021
2021/06/22 Letter of Announcement: IT Security EU Hub
22.06.2021
2021/06/22 Letter of Announcement: New Release of the EMVO Gateway: Confirmation of deployment
14.06.2021
2021/06/14 Letter of Announcement: New Release of the EMVO Gateway
07.06.2021
2021/06/07 Letter of Announcement: EU Hub Release 1.9: Confirmation of deployment to PRD
28.05.2021
2021/05/28 Letter of Announcement: Upload of data to the EMVS
26.05.2021
2021/05/26 Letter of Announcement: EU Hub Release 1.9: Deployment date for PRD
25.05.2021
2021/05/25 Letter of Announcement: Decommissioning of products: use of correct statuses
19.05.2021
2021/05/19 Letter of Announcement: EU Hub Release 1.9: Estimated deployment date for PRD
04.05.2021
2021/05/04 Letter of Announcement: EU Hub Release 1.9: Confirmation of deployment to IQE
03.05.2021
2021/05/03 Letter of Announcement: The Alert Management System (AMS) project – Technical Documentation and Q&As
30.04.2021
2021/04/30 Letter of Announcement: EU Hub Release 1.9: Confirmation of deployment to ITE
23.04.2021
2021/04/23 Letter of Announcement: EU Hub Release 1.9: Planned deployment dates for ITE, IQE & PRD
16.04.2021
2021/04/16 Letter of Announcement: EU Hub Release 1.9: Initial deployment dates for ITE, IQE and PRD
13.04.2021
2021/04/13 Letter of Announcement: Planned maintenance to the OBP Portal
01.04.2021
2021/04/01 Letter of Announcement: Exceptionally high number of alerts due to congestion in the EU Hub’s retry queue
05.03.2021
2021/03/05 Letter of Announcement: EMVO General Assembly meeting—Changes to EMVO’s Board of Directors and the reappointment of the NMVO Observers
24.02.2021
2021/02/24 Letter of Announcement: Archiving the backlog of A7 alerts (remaining in the retry queue from 2020)
22.02.2021
2021/02/22 Letter of Announcement: Clarifying the uploading requirements for packs intended for supply in Northern Ireland into the EMVS
15.02.2021
2021/02/15 Letter of Announcement: Update—Clearing the backlog of A7 alerts
09.02.2021
2021/02/09 Letter of Announcement: The European Medicines Verification System’s second anniversary
04.02.2021
2021/02/04 Letter of Announcement: Changes in the Enterprise Solutions (aka OPS) Team
02.02.2021
2021/02/02 Letter of Announcement: Keep informed about the latest EMVS incidents – subscribe EVI
27.01.2021
2021/01/27 Letter of Announcement: Increase in A7 alerts
25.01.2021
2021/01/25 Letter of Announcement: Delayed alerts propagation
01.01.2021
2021/01/01 Letter of Announcement: EU Hub inaccessible due to issues with the EU Hub endpoints addresses—ISSUE RESOLVED
01.01.2021
2021/01/01 Letter of Announcement: EU Hub inaccessible due to issues with the EU Hub endpoints addresses
23.12.2020
2020/12/23 Letter of Announcement: EMVO’s communication regarding the impact of Brexit and the Northern Ireland Protocol in the EMVS following the EU Commission’s Notice
15.12.2020
2020/12/15 Letter of Announcement: EMVO support and Helpdesk—Holiday season availability
15.12.2020
2020/12/11 Letter of Announcement: NMVO Fee Models Overview for 2021-2022
07.12.2020
2020/12/07 Letter of Announcement: EU Hub Release 1.8 deployment confirmation
30.11.2020
2020/11/30 Letter of Announcement: Renewal of the EU Hub’s security certificate
26.11.2020
2020/11/26 Letter of Announcement: Release 1.8–Change to “A99 – Possible counterfeit” code in the header
18.11.2020
2020/11/18 Letter of Announcement: EU Hub Release 1.8 deployment date for PRD
05.11.2020
2020/11/05 Letter of Announcement: EMVO’s communication regarding the impact of Brexit and the Northern Ireland Protocol in the EMVS
30.10.2020
2020/10/30 Letter of Announcement: EU Hub Release 1.8—Confirmation of deployment to IQE
27.10.2020
2020/10/27 Letter of Announcement: Reminder—Conforming to latest security standards—Transport Layer Security (TLS) 1.2 or higher
22.10.2020
2020/10/22 Letter of Announcement: Conforming to latest security standards: Transport Layer Security (TLS) 1.2 or higher
14.10.2020
2020/10/14 Letter of Announcement: Static IP addresses for the EU Hub – PRD deadline
06.10.2020
2020/10/06 Letter of Announcement: EU Hub Release 1.8—Confirmation of deployment to ITE
02.10.2020
2020/10/02 Letter of Announcement: EU Hub Release 1.8 Deployment dates for ITE/IQE and PRD
24.09.2020
2020/09/24 Letter of Announcement: Static IP addresses for the EU Hub—ITE deadline
17.09.2020
2020/09/17 Letter of Announcement: Reminder—Static IP addresses for the EU Hub
02.09.2020
2020/09/02 Letter of Announcement: Improving EMVO’s communication channels
10.08.2020
2020/08/10 Letter of Announcement: Update—COVID-19 measures for Belgium
30.07.2020
2020/07/30 Letter of Announcement: Static IP addresses for the EU Hub
16.07.2020
2020/07/16 Letter of Announcement: The Alert Management System (AMS) project
10.07.2020
2020/07/10 Letter of Announcement: InteliSecure’s IT Security Audit of the EU Hub
02.07.2020
2020/07/02 Letter of Announcement: Reminder — Support for the 2016 schema will end in Q4 2020
09.06.2020
2020/06/09 Letter of Announcement: Aggregation Projects — Guidelines for OBPs
27.05.2020
2020/05/27 Letter of Announcement: Enable EU Hub IP address in your firewall’s whitelist
20.05.2020
2020/05/20 Letter of Announcement: System disruption during EU Hub update
04.05.2020
2020/05/01 Letter of Announcement: EU Hub Release 1.7.02
30.04.2020
2020/04/30 Letter of Announcement: EMVS Alerts & Notifications document
29.04.2020
2020/04/28 Letter of Announcement: EU Hub Release 1.7.02
24.04.2020
2020/04/24 Letter of Announcement: Preliminary root cause analysis V.1.0
18.04.2020
2020/04/18 Letter of Announcement: EU Hub Release 1.7.01 – Complete
17.04.2020
2020/04/17 Letter of Announcement: EU Hub Release 1.7 Postponed
02.04.2020
2020/04/02 Letter of Announcement: Consequences of Data Withdrawal & Batch recall
01.04.2020
2020/04/01 Letter of Announcement: EMVO’s FMD Workshop in May
31.03.2020
2020/03/31 Letter of Announcement: COVID-19 Measures Update
30.03.2020
2020/03/30 Letter of Announcement: EU Hub Release 1.7 ITE & IQE confirmation
25.03.2020
2020/03/25 Letter of Announcement: OBPs using 2016 Schema
20.03.2020
2020/03/20 Letter of Announcement: EU Hub Release 1.7 ITE, IQE & PRD deployment
13.03.2020
2020/03/13 Letter of Announcement: COVID-19 Measures
11.03.2020
2020/03/11 Letter of Announcement: EU Hub Disruption Update
11.03.2020
2020/03/11 Letter of Announcement: European Commission Q&A Version 17
10.03.2020
2020/03/10 Letter of Announcement: EU Hub Disruption
06.03.2020
2020/03/06 Letter of Announcement: Master Data Maintenance – Versioning and Retrospective Upload
25.02.2020
2020/02/25 Letter of Announcement: Updated Fee Models
24.02.2020
2020/02/17 Letter of Announcement: EU Hub Release 1.7 information for OBPs
24.02.2020
2020/02/03 Letter of Announcement: Customer Support survey results
24.02.2020
2020/01/31 Letter of Announcement: OBP Portal User Interface (UI) back to normal
24.02.2020
2020/01/30 Letter of Announcement: OBP Portal User Interface (UI) not loading
24.02.2020
2020/01/20 Letter of Announcement: Retirement of the OBP Interface 2016 Schema
18.12.2019
2019/12/18 Letter of Announcement: New EMVO office address
13.12.2019
2019/13/12 Letter of Announcement: EMVO Helpdesk Christmas availability
26.11.2019
2019/26/11 Letter of Announcement: EMVO Helpdesk and Support
25.11.2019
2019/11/22 Letter of Announcement: OBP Portal Release 6.3
25.11.2019
2019/11/21 Letter of Announcement: Alert Messages: “manualentryflag” True/False
25.11.2019
2019/11/04 Letter of Announcement: EU Hub Release 1.6.02 deployment confirmation
25.11.2019
2019/10/31 Letter of Announcement: EMVO Gateway SSL certificate renewal
25.11.2019
2019/10/25 Letter of Announcement: EU Hub Release 1.6 PRD Deployment
18.10.2019
2019/10/18 Letter of Announcement: EU Hub Release 1.6 IQE deployment confirmation
11.10.2019
2019/10/10 Letter of Announcement: EU Hub Release 1.6 IQE deployment schedule
04.10.2019
2019/10/04 Letter of Announcement: Clarification, verifying one pack per batch
27.09.2019
2019/09/27 Letter of Announcement: Updated fee models
26.09.2019
2019/09/26 Letter of Announcement: Verifying one pack per batch
25.09.2019
09/10/2019 Letter of Announcement: Attempts to obtain sensitive information
25.09.2019
2019/09/25 Letter of Announcement: EU Hub 1.6
13.09.2019
2019/09/13 Letter of Announcement: EVI Maintenance
13.09.2019
2019/09/13 Letter of Announcement: Verification of packs not in hand
10.09.2019
2019/09/10 Letter of Announcement: EU Hub Release 1.6
05.09.2019
2019/09/05 Letter of Announcement: Data upload and verification lead time
03.09.2019
2019/09/02 Letter of Announcement: EU Hub ITE 1.4.08 restoration confirmation
29.08.2019
2019/08/29 Letter of Announcement: EU Hub ITE 1.4.08 restoration
21.08.2019
2019/08/21 Letter of Announcement: Alert message format
In order to ensure that OBPs can fully analyse alerts and their root causes, this LoA contains a translation table to compare the new alert message format to the previous one.

20.08.2019
2019/08/20 Letter of Annoucement: EU Hub Release 1.4.08 restoration – planned downtime
There will be a planned downtime of the EU Hub on Friday 23rd August from 13:00-17:00 (CET). In the meantime, the IQE environment cannot be considered as operable.

20.08.2019
2019/08/07 Letter of Announcement: Emergency fix for the EU Hub IQE environment
06.08.2019
2019/08/05 Letter of Announcement: EU Hub Release 1.5
We recently Deployed of EU Hub Release 1.5 in the IQE environment. However, we identified an issue with the renewal of session tokens in this environment. This issue was only detected once the release was deployed into IQE.
This means that currently the IQE environment is essentially offline and should not be used for testing and validation purposes.

26.07.2019
2019/07/26 Letter of Announcement: EU Hub Release 1.4.08
Release 1.4.08 of the EU Hub was successfully deployed to the Production environment at around 18:00 (CET), Thursday 25th July.

18.07.2019
2019/07/18 Letter of Announcement: Alert propagation
EMVO plans to propagate all alerts received from the National Systems to OBPs.

12.07.2019
2019/07/12 Letter of Announcement: EU Hub Release 1.5
EU Hub Release 1.5 has now been deployed in the IQE environment

01.07.2019
2019/06/28 | EU Hub Release 1.5 technical documentation
21.06.2019
2019/06/20 Letter of Announcement: EVI subscription
In this document you will find clear guidance on subscribing to notifications for national systems from the European Medicines Verification System Information (EVI) tool on the EMVO website.

20.06.2019
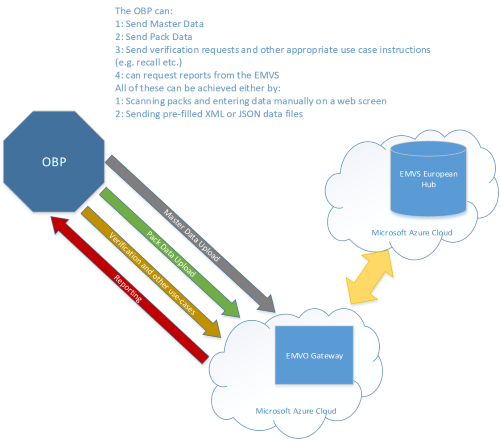
2019/06/2019 Letter of Announcement: Product Master Data Guidance
25.04.2019
2019/04/25| Letter of Announcement: 2016 schema retirement
01.02.2019
2019/02/01 Letter of Announcement: Downtime and Disruption Information System (DDIS) Update
19.12.2018
2018/12/19 | Downtime & Distribution Information System
19.12.2018
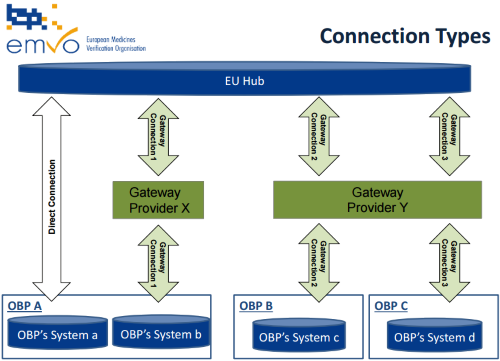
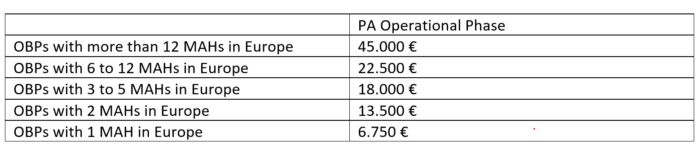
2018/12/19 | Participation Agreement, Operational Phase
18.12.2018
2018/12/18 | MAH Country Listing
23.11.2018
2018/11/23 Release 1.4.01 to PRD Scheduled
08.11.2018
2018/11/08 Letter of Announcement: re-scheduling of Release 1.4.01
06.11.2018
2018/11/06 | Participation Agreement for Operational Phase
06.11.2018
2018/11/06 | Letter of Adhesion
18.10.2018
2018/10/10 | MAH affiliation to the On-boarding Partner
10.10.2018
2018/10/10 | NMVS Functionality Matrix available
27.09.2018
2018/09/27 | EMVO notice of the new Participation Agreement
20.09.2018
2018/09/20 | Integrated Quality Environment of the EU Hub available
19.09.2018
2018/09/19 | Integrated Quality Environment of the EU Hub down
18.09.2018
2018/09/18 | New release of the EMVO Gateway
14.09.2018
2018/09/14 | Communication procedure update
11.09.2018
2018/09/11 | The Risk of the Falsified Medicines Directive Non-Compliance for your Business
06.09.2018
2018/09/06 | Product Pack Verification Functionality
03.09.2018
2018/09/03 | Retrospective Upload Guideline
20.08.2018
2018/08/20 | Successful deployment of the new Release of the EU Hub
16.08.2018
2018/08/16 | Summary of the Release 1.4 including the technical documentation
08.08.2018
2018/08/08 | EU Hub Alert on the Randomisation of serial numbers
07.08.2018
2018/08/07 | New Release of the EU Hub
06.08.2018
2018/08/06 | EMVO notice of On-boarding on time
03.08.2018
2018/08/03 | Complete your Contractual On-boarding in a timely manner!
24.07.2018
2018/07/24 | Modification of Marketing Authorisation Holder Information
20.07.2018
2018/07/20 |New EMVO Gateway Access Procedure
20.06.2018
2018/04/25 | Updated Master Data Guide
17.05.2018
2018/05/17 | On-boarding Fee Rise
09.05.2018
2018/05/09 | EU Hub Down for Maintenance
27.04.2018
2018/04/27 | NMVO’s Fee Models Overview on the EMVO Website
25.04.2018
2018/04/25 | Successful Hub Upgrade & Updated SDK Available
19.04.2018
2018/04/19 | EU Hub Down for Maintenance
12.04.2018
2018/04/12 | NMVOs Progresses in the On-boarding
04.04.2018
2018/04/04 | OBP Portal – New Release
16.03.2018
2018/03/16 | EMVO Notice to Future On-Boarding Partners
12.03.2018
2018/03/12 | 500 Participation Agreements signed!
02.03.2018
2018/03/05 | Momentary Unavailability of the German National System
27.02.2018
2018/02/27 | EMVO Website 2.0
22.02.2018
2018/02/22 | New version of .NET SDK available
09.02.2018
2018/02/09 | EMVO ON-BOARDING – Only one year left!
05.02.2018
2018/02/05 | Target Market for testing and certification
31.01.2018
2018/01/31 | Company Type
15.01.2018
2018/01/12 | Last Opportunity for a Timely On-boarding
15.01.2018
2018/01/08 | On-boarding Fee rise: A week left
15.01.2018
2018/01/04 | Technical On-boarding Training Video
15.12.2017
2017/12/15 | On-boarding Fee
08.12.2017
2017/12/06 | New version of .NET and Java SDKs
08.12.2017
2017/12/06 | New EU Hub Release 1.3
05.12.2017
2017/03/10 | Release of the OBP Portal
05.12.2017
2017/03/16 | New NMVO Section
05.12.2017
2017/03/16 | Training Video for the OBP Portal
05.12.2017
2017/07/24 | EU Hub 2 availability and New Release of the OBP Portal
05.12.2017
2017/07/24 | Updated Credential Handling for the OBP Portal
05.12.2017
2017/09/06 | EMVO On-boarding / NMVO Contracting
05.12.2017
2017/10/03 | EU HUB 2.0 and EMVO Gateway 3.0
05.12.2017
2017/10/04 | New release of OBP Portal